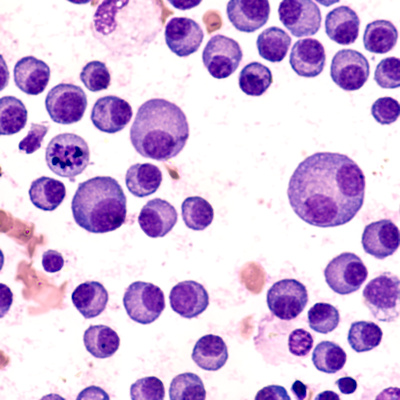
October 19, 2020 -- Oncopeptides has submitted an investigational new drug (IND) application to the U.S. Food and Drug Administration (FDA) for OPD5, a second drug candidate based on its proprietary peptide drug conjugate platform.
OPD5 is based on a proprietary platform that leverages aminopeptidases to release alkylating agents rapidly into tumor cells.
The company plans to initiate clinical development of OPD5 with an open label phase I dose escalation study on the safety and efficacy of OPD5 as a myeloablative regimen followed by autologous stem cell transplantation in patients with relapsed refractory multiple myeloma.
The first drug that Oncopetides has initiated regulatory activities for is melflufen.
Copyright © 2020 scienceboard.net
Member Rewards
Earn points for contributing to market research. Redeem your points for merchandise, travel, or even to help your favorite charity.
Research Topics
Interact with an engaged, global community of your peers who come together to discuss their work and opportunities.
Conferences
Connect
Tweets by @ScienceBoard