October 11, 2019 -- Scientists observed, for the first time ever, how variants of Parkinson's disease-associated protein alpha-synuclein change over time and identify the initial stages of protein aggregates. A new study published in Nature Communications on October 10 helps to clarify the why treatment challenges associated with Parkinson's disease (PD), when does it begin.
Researchers at the Federal University of Rio de Janeiro (UFRJ), Brazil, and the University of Virginia School of Medicine, USA worked to characterize and understand the organization of alpha-synuclein aggregates. Researchers already know that degradation of neurons leads to the onset of symptoms such as tremors and is linked to alpha-synuclein aggregates in the brain of PD patients. However, there is no consensus on what triggers the aggregation and degeneration, or how toxic the aggregates are to cells. This study explores these questions.
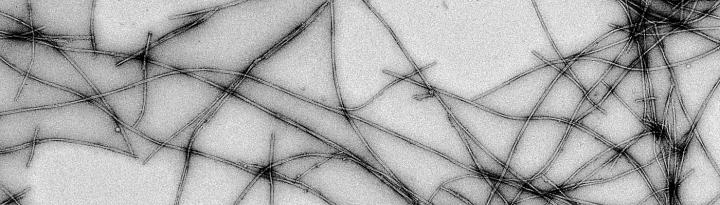
"A person develops Parkinson's disease over his lifetime. The conversion from one protein stage to the other takes place slowly. The intermediate structures and the amyloid aggregates accumulate over time in the brain. So far, we don't know which species cause the symptoms and toxicity to cells," explains the lead author of the research Guilherme A. P. de Oliveira, researcher at the University of Virginia and professor at the UFRJ. "If we understand the protein species forming during the early stages of disease conversion, we can propose new therapies for disease detection before the symptoms appear," he adds.
In the current research, scientists compared the conversion of four variants of alpha-synuclein over time (three linked to PD and one wild-type) in response to ionic strengths. The team performed a battery of biochemical, kinetic, and structural studies to understand the early stages of alpha-synuclein polymerization and the polymorphism of heritable alpha-synuclein variants. They observed significant differences in the aggregation processes of each protein and found that oligomers develop at a much greater rate in early onset cases than in aging cases of PD. Such results may explain the early onset of symptoms in patients bearing these variants.
They also determined which proteins are most important for amyloid filament growth, leading to aggregation. These filaments have distinct structures, particularly length and conformation, based on the protein mutation from which they originate.
"Most intriguing is that not only the initial association steps are different, but also some mature filaments of hereditary cases. These filaments can twist differently depending on which mutation is present," explains Jerson Lima Silva, second co-author and professor at UFRJ.
Cutting-edge bio-imaging were used in this study. Modified fluorescent probes were used to detect intermediate species participating on alpha-synuclein association. Cryo-electron microscopy was used to observe the structural organization of alpha-synuclein. "By plunge freezing these samples and acquiring advanced electron microscope images, we are able to better understand these wrong protein associations in their native environment and ways to avoid their formation. I am glad that Brazil is now making part of this S&T venture," celebrates Oliveira.
Do you have a unique perspective on your research related to bio-imaging? The Science Advisory Board wants to highlight your research. Contact the editor today to learn more.
Copyright © 2019 scienceboard.net






