March 16, 2020 -- A new simple and scalable protein-processing technique based on temperature-sensitive phase changes creates unique microarchitectures within microparticles for use in drug delivery and other bioengineering applications, according to a study published in Nature Communications on March 12.
Novel microstructures with complex architectures and spatially segregated regions are needed for materials science and bioengineering applications. The function of these biocompatible microparticles is driven by their size and shape, internal microstructure, and the properties of their components. Currently, biocompatible microparticles are being used in these areas, but they often require sophisticated manufacturing techniques and the vast majority are composed of synthetic polymers or biologically derived polysaccharides.
Fabrication by multiple-emulsion microfluidics enables tight control over individual oil droplets but struggles to keep materials completely separate from one another and cannot be used for large-scale production. Alternatively, flow lithography, which shines light through a patterned mask to etch shapes in soft materials, can make many particles quickly but is difficult to tailor to complicated shapes and internal architectures.
Therefore, new microarchitectures based on alternative, biologically relevant materials produced in a safe and efficient manner are greatly needed. Biomedical engineers from Duke University joined forces to develop these biological materials. The team used a combination of elastin-like polypeptides (ELPs) and partially ordered proteins (POPs) to create unique particle shapes.
Elastin-like polypeptides (ELPs) are synthesized biopolymers that derive their stability from chaos and have no true shape. These disordered, thermoresponsive proteins shift between phase states at certain temperatures that can be controlled. Partially ordered proteins (POPs) integrate ELPs with ordered polyalanine helices. POPs exhibit thermal hysteresis -- the difference between transition temperatures defined during heating and cooling processes -- to trigger the formation of porous, physically crosslinked viscoelastic networks.
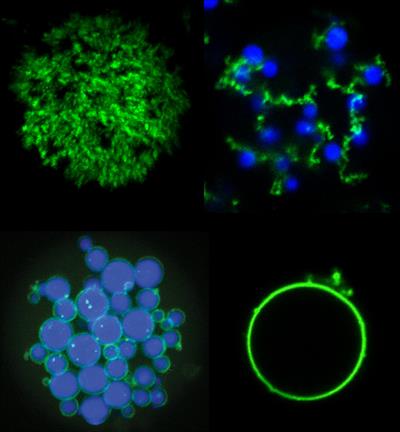
"Disordered proteins are a hot topic in biology, with many researchers trying to discover how proteins without shape can still have a biological purpose," said Stefan Roberts, PhD, biomedical researcher at Duke, in a university statement. "An undercurrent of our work is to instead think of these proteins as a materials scientist would and see if we can engineer them for our own biological functions in ways that can't be achieved with current materials."
In the study, researchers combine mixtures of the two types of proteins to create complex microarchitectures using only simple droplet microfluidics and stepwise heating and cooling. Through a series of heating steps, the combination of ELPs and POPs caused the formation of "fruits" of ELP on a POP network "vine" in solution. In a different set of temperature changes, heating POPs above their threshold temperature, they form physically crosslinked interconnected porous shells around ELP droplets.
To protect the stability of the microstructures, unnatural amino acids (UAAs) were incorporated as extrinsic crosslinking agents upon exposure to ultraviolet (UV) light. xPOPs, or POPs that include evenly spaced para-azidophenylalanine (pAzF) residues, photochemically react after short UV exposure to form crosslinks during heating. Subsequent cooling does not resolubilize the microparticles, like non-UAA POPs.
For many downstream processes, it is often necessary to extract the POP microparticles back into an aqueous environment from the emulsion. This was achieved with simple demulsification processing using the UAAs, which allowed for the recovery of stable, nonaggregating microparticles.
From a manufacturing perspective, each system the researchers designed simultaneously creates millions of solid, biocompatible microparticles slightly larger than an average cell. It only takes a few minutes, and it all happens in a volume of liquid about the size of a drop of water.
"This is a test case for a type of material that is flexible and simple enough to create both commonly used shapes and architectures that aren't seen using current techniques," Roberts said. "We're using new biocompatible materials to create never-before-seen shapes simply by heating, cooling, and shining a light on them."
Do you have a unique perspective on your research related to drug delivery systems and bioengineering? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net






