September 16, 2020 -- Researchers have identified a novel approach to treating SARS-CoV-2 infection, spurred by a new understanding of viral attachment involving a heparan sulfate, a common glycoprotein. The results are presented in a September 14 article published in Cell.
Some viruses interact with heparan sulfate, a highly negatively charged linear polysaccharide that is attached to a small set of membrane or extracellular matrix proteoglycans. In this format, glycan-binding domains on membrane proteins facilitate initial attachment of virions to glycan receptors, leading to eventual virus engulfment and internalization.
Trimeric spike proteins of SARS-CoV-2 engage human angiotensin-converting enzyme 2 (ACE2) with one or more receptor binding domains (RBD) in the "open" active conformation. Adjacent to the ACE2 binding site and exposed in the RBD is a group of positively charged amino acid residues that can potentially interact with heparan sulfate or heparin (a highly sulfated form mainly found in mast cell secretory granules used as an anticoagulant).
Heparan sulfate is a cofactor for SARS-CoV-2 cell binding
Researchers from the University of California (UC) San Diego School of Medicine developed a map of the SARS-CoV-2 RBD that revealed a heparin/heparan sulfate (H/HS) binding site. Docking studies demonstrated that the site could accommodate a chain of up to 20 monosaccharides.
Importantly, they found that the site is adjacent to but separate from the ACE2 binding site and that the RBD could simultaneously bind both cell surface heparan sulfate and ACE2 protein receptor.
The simulations indicate that the H/HS binding site is partially obstructed in the "closed" inactive RBD conformation, and fully exposed in the open state. Although the H/HS binding site is somewhat occluded in the closed conformation, it can still bind heparin albeit at a lower affinity.
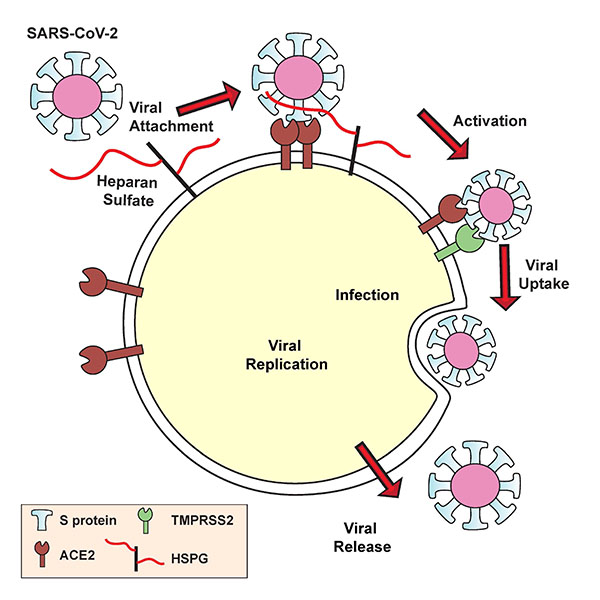
Furthermore, the researchers confirmed that heparin binding increases the open conformation of the RBD and thus increases binding to ACE2. To this end, the team explored spike reliance on cellular heparan sulfate for binding using heparin lyases to degrade cell surface heparan sulfate and CRISPR/Cas9 to inactivate an enzyme that is required for the synthesis of the heparan sulfate backbone. The absence of heparan sulfate essentially eliminated cell binding of the RBD at the H/HS binding site.
The findings identify cellular heparan sulfate as an essential cofactor that forms a ternary complex with ACE2 and RBD during SARS-CoV-2 infection.
Therapeutic approaches to using heparin
This new understanding of the viral mechanism of SARS-CoV-2 infection revealed heparan sulfate-spike protein complexes as a novel therapeutic target to prevent infection. The researchers already determined that removing heparan sulfate with enzymes blocked SARS-CoV-2 from binding to ACE2. From a therapeutic approach, they also hypothesized that heparin can be used as bait to lure and bind the coronavirus away from human cells.
Therapeutic unfractionated heparin, nonanticoagulant heparin, and heparan sulfate derived from human tissues were evaluated for their ability to block binding of the spike protein to ACE2. Unfractionated heparin and low molecular weight heparin are commonly used in the clinic to treat COVID-19 patients who suffer from thrombotic complications, according to the authors.
Unfractionated heparin effectively blocked infection of cells by spike protein pseudotyped virus and authentic SARS-CoV-2 (Vero E6 cells) without negatively impacting ACE2 expression or normal cell viability. This points to the potential to use unfractionated heparin to prevent viral attachment.
While these results are promising, the authors noted that a heparin-based therapy for COVID-19 is still far off. Researchers will need to test heparin and heparan sulfate inhibitors in animal models of SARS-CoV-2 infection. Other researchers at UC San Diego are exploring the role of the microbiome in modulating heparan sulfate, thus influencing a person's susceptibility to COVID-19.
"All of the things we've learned about heparan sulfate and the resources we've developed over the years have come together with a variety of experts across multiple institutions who were quick to collaborate and share ideas," explained Jeffrey Esko, PhD, distinguished professor of cellular and molecular medicine at UC San Diego School of Medicine and co-director of the Glycobiology Research and Training Center, in a statement. "If there's a silver lining to this pandemic, I hope it's that the scientific community will continue to work rapidly together like this to address other problems."
Do you have a unique perspective on your research related to virology or infectious disease research? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net


