October 4, 2019 -- Researchers at Oregon Health & Science University used cryoelectron microscopy (cryo-EM) to observe protein structure and function at a molecular level. This discovery, published in Cell on October 3 describes the structure of P2X receptor, a cellular membrane protein receptor, and provides insight into how the cation channel functions.
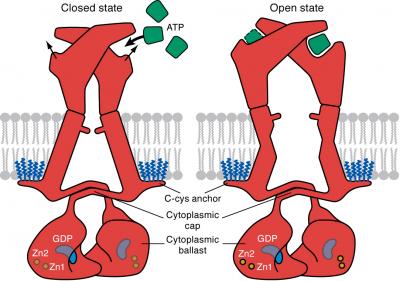
P2X family of receptor are ligand-gated ion channels that have been associated with inflammation, plaque buildup in arteries, cancer metastasis, neurological conditions, and other conditions. P2X receptors are considered therapeutic targets because their activation by ATP is a key step in the release of inflammatory cytokines. Many pharmaceutical companies have developed small molecules to selectively block the receptors. P2X7 receptor, which was specifically investigated in the current research, is unique because once activated it remains open indefinitely, this allows charged particles to enter a cell and trigger signaling pathways at will. The resultant uninhibited signaling behavior can contribute to any number of ailments associated with illness.
Using cryo-EM the research team was able to visualize individual units within the receptor that are located within the cell. When the receptor is in apostate (unbound) the team determined that the receptor is closed however when it is in the ATP-bound state it is open. Elucidating the internal structure further, scientists found that the cytoplasmic element, C-cys anchor, prevents desensitization by anchoring pore-lining helix to the membrane with palmitoyl groups. If these fatty acids are removed the receptor closes, shutting down its ability to trigger signaling pathways.
"Researchers have known ligand-gated ion channels are modified by palmitoyl groups, but we had never directly observed it until now," said Steven Mansoor, MD, PhD, assistant professor of medicine (cardiovascular) in the OHSU School of Medicine and Knight Cardiovascular Institute. "Our finding could be used as a model of how palmitoylation modifies other ion channels."
The researchers also unexpected discovered a second cytoplasmic element with a unique fold, termed a cytoplasmic ballast, which contains a zinc ion complex and guanosine nucleotide-binding site.
The team will further explore the roles of this guanosine nucleotide and the fatty acid groups in P2X7 intra-cellular signaling and potential impacts on human health. The new information provided by this research could inspire researchers to develop new drugs to treat inflammation, coronary artery disease, cancer, multiple sclerosis, and many other inflammatory diseases. They intend to leverage this knowledge to develop targeted therapies exploiting the function of P2X receptors.
This research was supported by the National Heart, Lung and Blood Institute (grant K99HL138129). The team used microscopes at the Pacific Northwest Center for Cryo-EM, which OHSU and Pacific Northwest National Laboratory established in 2018 with support of the National Institutes of Health, and conducted research in the OHSU Vollum Institute lab of Eric Gouaux, Ph.D., who is supported by the Howard Hughes Medical Institute.
Do you have a unique perspective on your research related to cell biology or human diseases? The Science Advisory Board wants to highlight your research. Contact the editor today to learn more.
Copyright © 2019 scienceboard.net


