January 21, 2021 -- Using an engineered DNA therapeutic agent to silence transcription factor genes reduced myeloma stem cell abundance and increased the survival of mice bearing human myeloma, according to preclinical study data published in Cell Stem Cell on January 20. The results of the study have prompted the clinical investigation of a therapeutic candidate for the treatment of multiple myeloma.
Multiple myeloma is the second most common blood cancer in the U.S. It generally recurs and is refractory (reoccurs and is unresponsive to treatments). Despite novel therapies, treatment toxicities continue to be a challenge for patients and clinicians.
In multiple myeloma, proinflammatory cytokines and antiviral interferons derived from bone marrow play a key role in disease progression, including activation of interleukin 6 (IL-6) with downstream interferon-response factor (IRF) signaling.
IRF4 has been determined to be an essential multiple myeloma cell survival factor with aberrant activation during pathogenesis and progression of the disease. In multiple myeloma, high IRF4 expression is also associated with lower overall survival rates.
IRF4 is known to drive expression of stem cell reprogramming genes such as the oncogenes MYC and KLF4. Yet the role of IRF activation in the maintenance of malignant progenitors in multiple myeloma has not been explore. Therefore, the researchers hypothesized that IRF4 governs multiple myeloma progenitor regeneration to promote disease progression.
Because transcription factors (such as IRF4) are difficult to target with traditional small-molecule strategies, researchers from the University of California (UC) San Diego School of Medicine and Ionis Pharmaceuticals used an antisense oligonucleotide-based inhibitory platform to reduce the stability of IRF4 transcripts for the purpose of disrupting cancer metabolism.
Preclinical evaluation of antisense oligonucleotides
The researchers tested a panel of human-specific antisense oligonucleotide agents targeting IRF4 in vitro and profiled their effects on multiple myeloma cell viability and IRF4 expression to determine a candidate that selectively inhibits human IRF4. The antisense oligonucleotides dramatically reduced survival of multiple myeloma cells and decreased human IRF4 messenger RNA (mRNA) and protein expression, as well as lowered expression of the IRF4 target gene MYC.
To confirm their results, the team conducted ex vivo treatments using patient-derived xenograft human multiple myeloma cells. The cells were sensitive to the treatment with significant reductions in cell viability and IRF4 mRNA expression.
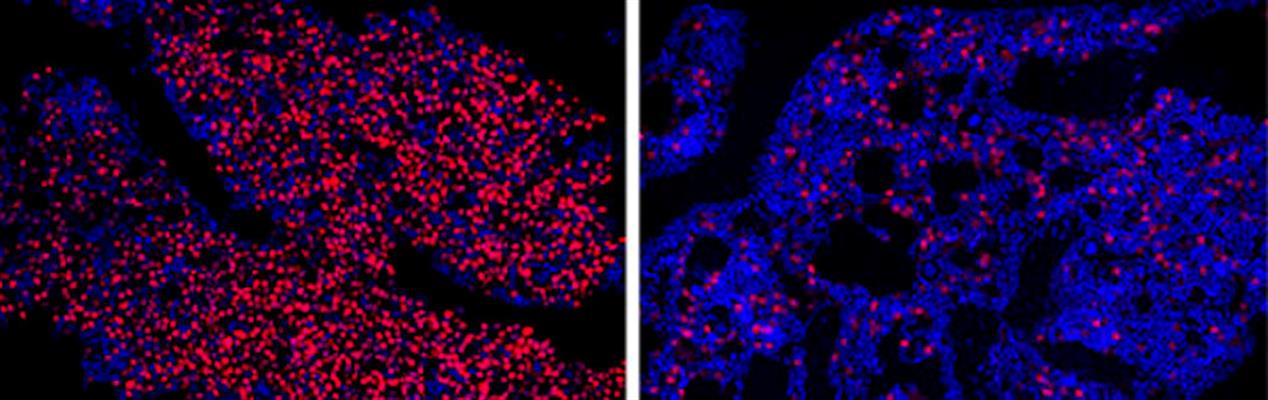
In xenograft mouse models, the treatment was well tolerated in myeloma tumor-bearing mice and significantly reduced tumor growth and IRF4 mRNA expression in tumor tissues in a dose-dependent manner after two to six weeks of treatments. The authors noted that 70%-100% of treated mice survived, compared to none in the untreated control group of mice.
"The results of these preclinical studies were so striking that half the microscopy images we took to compare bone marrow samples between treated and untreated mice kept coming back blank -- in the treated mice, we couldn't find any myeloma cells left for us to study," said co-senior author Leslie Crews, PhD, assistant professor in the Division of Regenerative Medicine at the UC San Diego School of Medicine, in a statement. "It makes the science more difficult, but it gives me hope for patients."
The researchers observed a potent on-target effect of the agent in degrading IRF4 transcripts and reducing protein production in myeloma-relevant tissues. Importantly, they found that treatment reduced the expression of IRF4 target genes, as well as other microenvironment-responsive and cell cycle regulatory transcripts but spared normal hematopoietic cells.
The researchers noted that one benefit of antisense oligonucleotides is that they can be delivered into cells by free update with natural endocytic mechanisms, which enables rapid gene expression modulation compared to alternative gene delivery strategies. Additionally, the treatment improved myeloma tumor cell sensitivity to standard-of-care cancer therapeutics such as lenalidomide therapy.
"These proof-of-principle studies will enable rapid clinical development of antisense oligonucleotide-mediated IRF4 inhibition to prevent myeloma relapse driven by drug-resistant cancer stem cells," said co-senior author Dr. Catriona Jamieson, PhD, and director of the California Institute for Regenerative Medicine (CIRM) Alpha Stem Cell Clinic at UC San Diego Health.
The team concluded that comprehensive preclinical studies showed that blocking human IRF4 with selective antisense oligonucleotide agents potently inhibits malignant multiple myeloma stem cell survival and regeneration while sparing normal stem cells and immune cell development.
The studies also led to the identification of a lead antisense oligonucleotide candidate for clinical development (ION251) providing a path forward for clinical evaluation in human trials targeting IRF4. The phase I clinical trial to assess the safety of ION251, sponsored by Ionis Pharmaceuticals, is now recruiting participants at Moores Cancer Center at UC San Diego Health and elsewhere.
Do you have a unique perspective on your research related to cancer research or drug development? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net


