February 10, 2021 -- Bioengineers have developed a new way to engineer the human epigenome (chemical changes in the DNA) using a modified CRISPR-Cas9 system to target and activate proteins in the chromosome. This research, published in Nature Communications on February 9, expands on synthetic genome tools.
DNA methylation and posttranslational modification to histones play a large part in controlling human gene expression. There are four chromatin proteins, called histones, in each nucleosome (a structural unit of the chromosome) that help control the structure and function of genomes by exposing genes for activation.
"Nucleosomes serve as architectural substrates to fit our DNA inside of our cells and can also control access to key parts of our genomes," said senior author Isaac Hilton, PhD, an assistant professor at Rice University, in a statement.
Histone phosphorylation, the addition of a phosphoryl group, can control protein-to-protein or protein-to-DNA interactions. For instance, histone phosphorylation at serine residue 10 and 28 on histone subunit H3 (H3S10ph and H3S28ph) are types of posttranslational modifications tied to stimulus-dependent gene expression. However, defining the causal role has been difficult due to lack of available technology.
"Histones can display an exquisitely diverse spectrum of chemical modifications that serve as beacons or regulatory markers and tell which genes to turn on, and when, and how much to do so," Hilton said. "One of these mysterious modifications is phosphorylation, and we aimed to better illuminate the mechanism by which it can rapidly turn human genes on and off."
Building the search tool
The mitogen- and stress-activated protein kinase 1 (MSK1) is one of nine human proteins known to catalyze H3S10ph and H3S28ph in vitro. MSK1 is known to control RNA polymerase II-mediated transcription in human cells. Furthermore, MSK1-driven H3S10ph or H3S28ph has been correlated with the transactivation of stimulus-responsive genes through chromatin immunoprecipitation (ChIP) assays.
With this in mind, Rice University bioengineers constructed a fusion protein, called dCas9-dMSK1, that consists of a deactivated Cas9 protein from Streptococcus pyogenes (dCas9) and a hyperactive variant of human MSK1. The deactivated Cas9 protein, borrowed from the CRISPR-Cas9 system, allows for specific targeting -- but not cutting -- of targeted sequences. The dCas9-dMSK1 tool uses a recruited dMSK1 enzyme to phosphorylate targeted histones and turn on nearby genes.
The researchers started by identifying if the dCas9-dMSK1 system could modulate the phosphorylation and/or gene expression of natural MSK1 targets. They found dCas9-dMSK1 can activate BMP2 and GDF6 (direct targets), but not PRKCB, SHROOM2, and ZNF462 (indirect targets of MSK1). Further investigation with ChIP-quantitative polymerase chain reaction (qPCR) found that MSK1 was significantly enriched in the promoter regions of the direct targets.
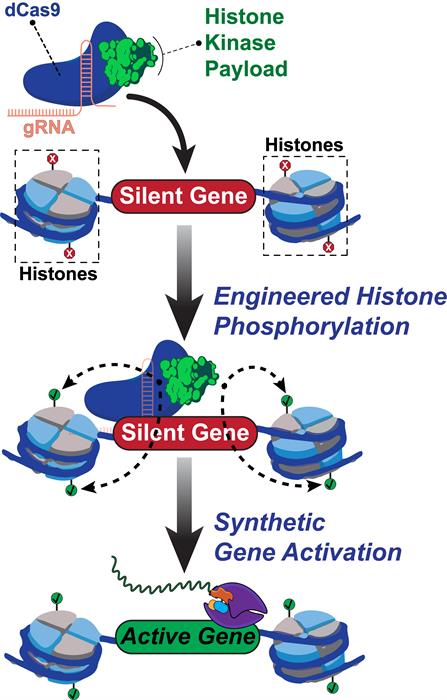
Within the promoter region, the team found that dCas9-dMSK1 efficiently phosphorylated both H3S10 and H3S28 compared to the control. Cumulatively, the authors suggested that the data indicate targeted, locus-specific activation of gene expression from human promoters that are sensitive to histone phosphorylation.
Next, the researchers examined if the dCas9-dMSK1 tool could activate other promoter regions with specificity. They found that dCas9-dMSK1 was able to specifically bind and activate promoters across the human genome (e.g. octamer-binding transcription factor 4 [OCT4] promoter) and transcriptome (e.g. OCT4 protein isoforms). Moreover, dCas9-dMSK1 resulted in increased H3S10ph and H3S28ph levels at these targeted promoters. The engineers showed that this capability is functional at diverse human promoters and in different human cell types and can be achieved using other histone kinases.
The research also suggests that histone phosphorylation driven by dCas9-dMSK1 is influenced by, and can influence, the dynamics of surrounding histone posttranslational modifications. For instance, targeting dCas9-dMSK1 to the OCT4 and myoblast determination protein 1 (MYOD) promoters resulted in increased acetylation status of histone H3 lysine 27 (H3K27ac) levels at both loci.
"It tells us that chemical modifications on histones talk to each other, and we can show it happening at specific spots in the human genome," said lead author, Jing Li, PhD, a member of the Hilton lab at Rice University. "And that's linked to a gene turning on, so this allows us to synthetically control them."
Using the tool to screen for therapeutic resistance
Lastly, the Hilton lab used dCas9-dMSK1 to uncover novel genes and pathways associated with drug resistance. They showed that the system can be an unbiased approach to screen the noncoding genome in a high-throughput manner.
They combined dCas9-dMSK1 with a genome-scale guide RNA library to identify genes that when synthetically overexpressed by dCas9-dMSK1, would result in resistance to PLX-4720, a BRAF V600E inhibitor, in A375 melanoma cells with a dCas9-dMSK1 lentiviral expression vector. The cells were transduced with a CRISPR activation guide RNA library and subjected to a control or PLX-4720 treatment for 16 days. Next-generation sequencing was used to reveal enriched guide RNA sequences.
The team identified three of the top 10 genes identified as mediators of PLX-4720 resistance (EPDR1, AFF2, and ERC2) were previously associated with melanoma drug resistance. The additional seven (MRPS15, TRAT1, LACC1, AGL, TDRP, MIPOL1, and LELP1) are new genes linked to melanoma resistance.
"It's a complicated story," said Li. "There are a lot of different positions and features of histones that we want to study."
Do you have a unique perspective on your research related to synthetic biology? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net


