June 4, 2021 -- A new gene engineering technology could allow scientists to harness the benefits of releasing genetically modified animals into the wild without the risk of uncontrolled spread. The new study, published in the journal Nature Communications on June 2, could help in the battle against the spread of diseases like malaria.
The advent of the genetic age offers the tantalizing prospect of being able to genetically alter animals, such as pests and disease vectors, to reduce the harm they cause to society. However, any technology with the ability to make a difference on a significant scale would also have the potential to cause serious damage if it went out of control.
Gene drives are one such technology. These genetic modifications are designed to spread through a population quickly and rely on the CRISPR-Cas9 gene editing system to make a duplicate copy of the gene drive on the partner chromosome. This means that all offspring inherit the gene, compared to only 50% through normal genetic inheritance.
However, there are concerns about the effect of releasing such gene drives into the wild. Unintended consequences, potentially due to mutations or ecological shifts, could be irreversible.
This has led geneticists to search for new versions of gene drives that can prevent unrestricted spread by stopping engineered animals from crossbreeding with the wild population. Approaches previously developed have severe limitations, such as not working in multicellular organisms, causing high fitness costs, or working incompletely.
A way to control populations
Now, geneticists have developed a new approach called Synthetic Postzygotic barriers Exploiting CRISPR-based Incompatibilities for Engineering Species (SPECIES) that prevents crossbreeding. SPECIES uses an adapted form of the Cas9 protein that is used to target gene sequences in the CRISPR editing technique. The adapted Cas9 recruits the cell's transcriptional machinery to genes essential for development, causing the cell to overexpress the gene to a lethal degree.
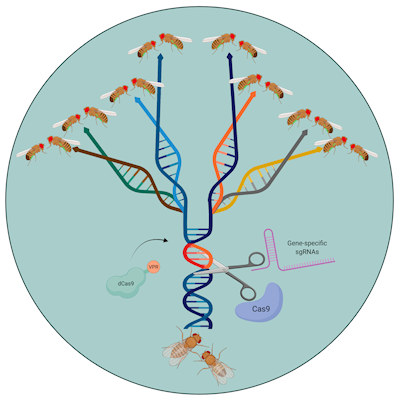
The researchers can prevent this lethal overexpression in the engineered SPECIES organisms by mutating the genes' promoter regions to stop Cas9 binding. If an engineered animal attempted to breed with a wild type, the offspring would not survive as the Cas9 would target the wild-type development gene, triggering overexpression.
"Gene drives can potentially spread beyond intended borders and be hard to control. SPECIES offers a way to control populations in a very safe and reversible manner," said Omar Akbari, PhD, an associate professor at the University of California, San Diego Division of Biological Sciences and senior author of the paper, in a statement.
In the new study, the team described how it generated eight engineered SPECIES of fruit flies that could not breed with one another or with the wild-type fly, Drosophila melanogaster. By targeting two to three genes per SPECIES, the researchers hope this will ensure that naturally occurring mutations will not allow crossbreeding.
The approach reflects the natural formation of new species, where separate populations of the same species gradually evolve divergently until their genetics are no longer compatible for breeding.
"Even though speciation happens consistently in nature, creating a new artificial species is actually a pretty big bioengineering challenge," said Anna Buchman, PhD, a researcher in Akbari's lab and the lead author of the paper. "The beauty of the SPECIES approach is that it simplifies the process, giving us a defined set of tools we need in any organism to elegantly bring about speciation."
Harness the power
One of the most sought-after targets for gene drive technology is to prevent the spread of malaria through mosquitoes. Genetically modified mosquitoes that cannot carry the disease could be used to overwhelm the wild population and halt its spread.
As the SPECIES gene drive cannot proliferate through breeding with wild-type animals, an engineered SPECIES needs to be released repeatedly to dominate the population. If the new SPECIES exceeds a certain threshold percentage of the overall population, it can replace the wild type.
However, below this threshold, the wild type can take over again if the releases stop. This is because the wild-type animals would have a reproductive advantage over the engineered SPECIES due to small fitness costs caused by the system.
"Since SPECIES are incompatible with wild-type mosquitoes, their populations can be controlled and reversed by limiting their threshold population below 50%. This gives you the ability to confine and reverse its spread if desired," Akbari said. "This essentially allows us to harness all of the power of gene drives -- like disease elimination or crop protection -- without the high risk of uncontrollable spread."
Do you have a unique perspective on your research related to synthetic biology? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net