January 13, 2021 -- 2020 was a great year for artificial intelligence (AI) in drug development, where the total investment in the industry hit $9.2 billion and many partnerships between AI specialists and the pharmaceutical industry were formed. 2021 may prove to be just as exciting, as this year's first acquisition was already confirmed when Charles River Laboratories announced the acquisition of Distributed Bio for $83 million plus $21 million in performance payments.

How the AI drug development vendors are organized
There are some 250 AI companies that target drug development, and they are distributed across the development stages from early drug discovery to late clinical trial optimization. Most vendors have a very specific therapeutic focus in a defined stage of the development process, whereas others build platforms serving across development stages and therapeutic areas.
Early-stage drug design vendors use AI applications to develop new molecules, predict and optimize drug specificity and efficacy, or select drug repurposing options for existing drugs. Vendors focusing on late-stage development use AI applications to optimize the clinical trial process by improving patient stratification, optimizing enrollment and retention, and helping to reduce the number of patients needed for a successful trial.
Working across the entire drug development process -- but often used in clinical trials -- are AI platforms called information engines. These AI platforms aggregate and analyze information and real-world evidence from multiple sources such as scientific literature, patient data, and clinical trial information to find new associations and guide drug discovery or clinical trial optimization.
The pharma-AI partnership model
Business models covering the pharma-AI partnership vary greatly across the industry, but generally fall into distinct categories depending on the type of application being offered. Particularly for AI applications in drug discovery and design, revenues are split between an initial payment upon signing the agreement and performance-dependent milestone payments as the projects progress. These milestone payments are often several times larger than the initial payments.
For most AI specialists, this provides revenue to help cover some of their operational costs, which helps them look more favorable to future investors, and it gives promises of rapid financial growth once projects are successfully completed. For pharma partners, the partnership model reduces the risk associated with drug development and forms the foundation for tighter relationships with successful AI specialists.
The emerging role of CROs in the AI ecosystem
Until now, drug discovery AI startups have mainly earned their revenue from partnerships with pharma companies. But while contract research organizations (CROs) have traditionally acted merely as a bridge between startups and the pharma sponsor in clinical trials, some CROs are now looking at AI startups as components that could add considerable value to their offering.
While the capabilities of most traditional CROs are shifted toward late-development stages and clinical trials, an increasing number of CROs are now specializing in drug discovery services, either as a sole focus or with an ambition to offer end-to-end services to the pharmaceutical industry. For these CROs, AI drug discovery startups are particularly interesting because of the following:
- They support their reputation as progressive drug discovery partners with capabilities beyond traditional pharma companies.
- They can use their capabilities across different projects with multiple pharma partners and therefore add significant value.
- They help CROs acquire AI competencies, which can create synergy with other business units within the CRO.
- They strengthen the overall end-to-end offering to create tighter partnerships with pharma companies.
For AI startups being acquired by a CRO, it can be an attractive exit strategy too. They can continue their existing partnerships with pharma companies and will remain in a similar consultative role serving the pharma industry as a whole, as opposed to being restricted by the pipeline limitations of a pharma acquirer. They will keep the potential to add value to projects across the industry and benefit from the CROs established pharma partnerships.
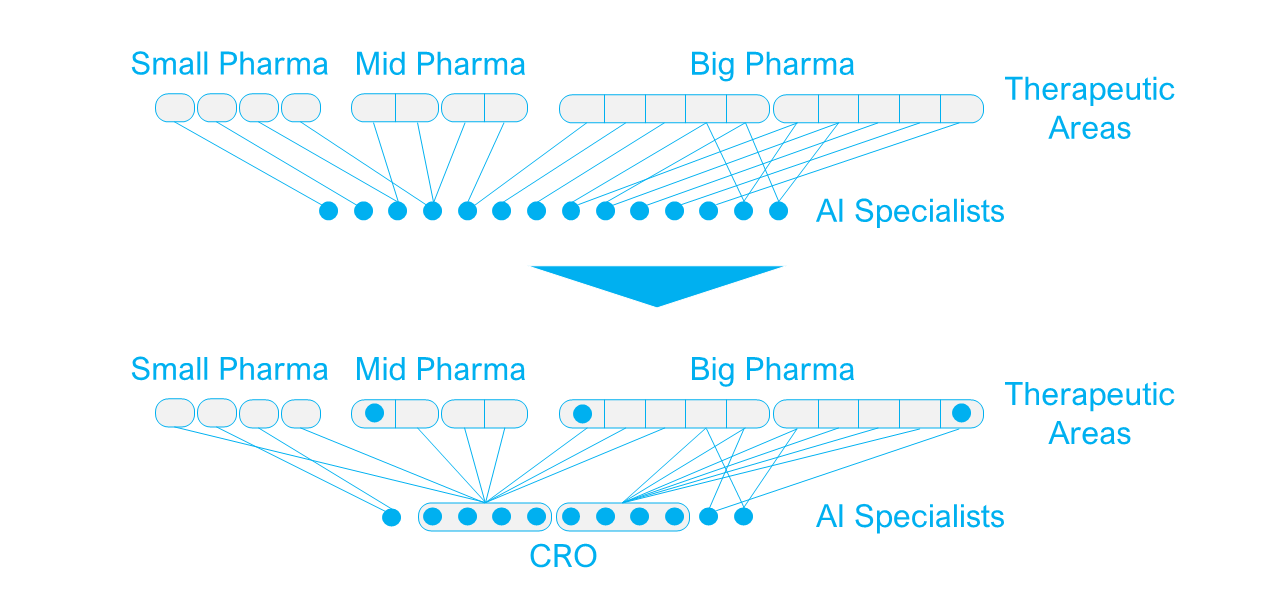
The emerging role of CROs in the AI ecosystem. Image courtesy of Emersion Insights.
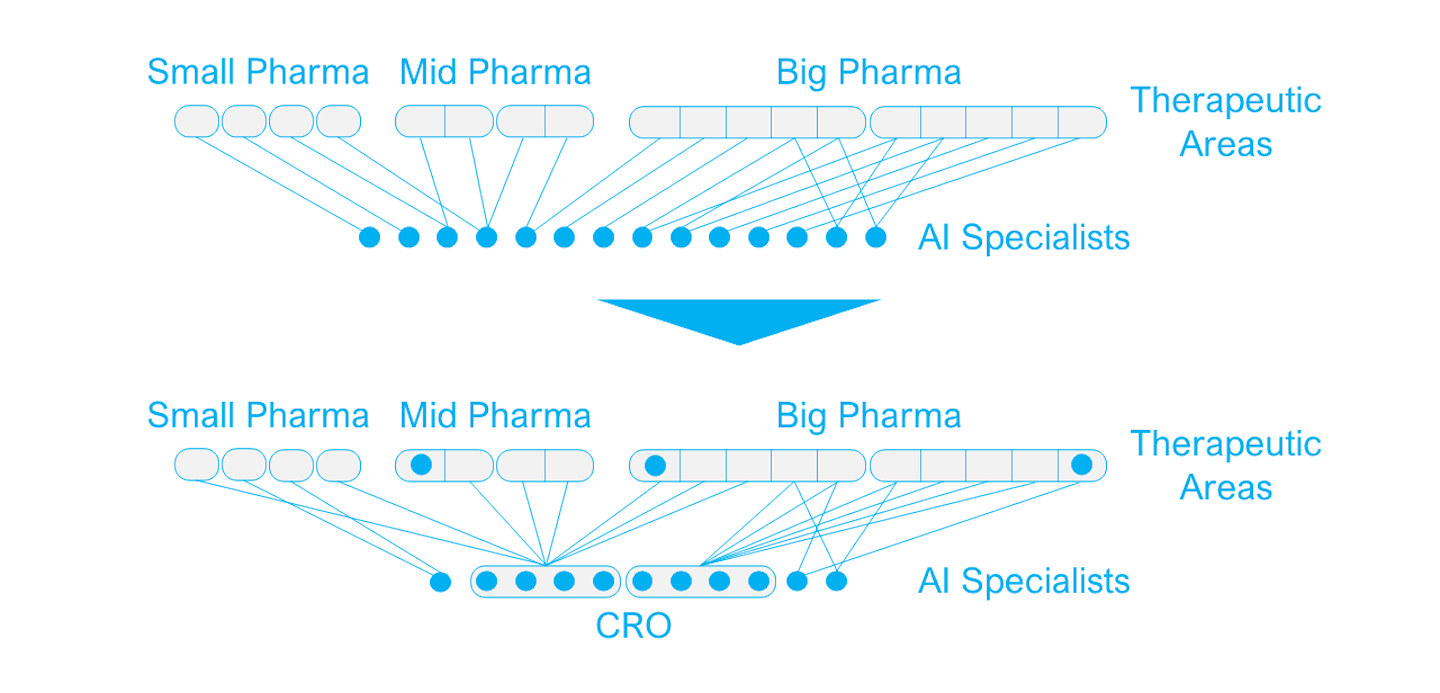
The emerging role of CROs in the AI ecosystem. Image courtesy of Emersion Insights.
How will the pharma industry react?
For the pharmaceutical industry, the rise of AI in drug development is no surprise and actually plays well with their long-term strategy of reducing cost and risk in their R&D programs, while remaining competitive. The current milestone partnership model is exactly what the pharma industry needs to increase profitability in their R&D programs, since only successful projects impose notable expenses. So for a pharmaceutical company, an AI specialist being acquired by a CRO represents an AI specialist that's not being acquired by a competitor, and therefore a company that can remain a long-term partner.
However, looking ahead, we will start to see pharma companies acquiring AI partners that are essential to their main therapeutic areas. These acquisitions will be motivated by a wish to strengthen internal R&D capabilities to secure future competitiveness in a more cost-effective way, but also to prevent an essential partner from being acquired by a competitor. The acquirers will be aware that these acquisitions introduce risks and costs they would have preferred to be without, and they will therefore hold off until the technologies have proven their worth on ongoing projects and they could risk losing the AI partner to a competitor.
Over the next three to five years, milestone payments to AI partners will increase significantly as projects come to completion. In the meantime, pharma companies are focusing on developing their AI infrastructure and creating a framework that maximizes the value of their AI efforts.
This means they are currently engaged in hefty digital transformations to open up siloed data and build compatibility across functions and businesses. They thereby create large quantum computing-ready data lakes that can serve as a foundation for AI-enabled drug development as well as business process optimizations.
For an AI specialist to add significant value to a pharma company through acquisition, it will need to become an integrated component in these efforts.
Ulrik Kristensen, PhD, is founder and principal analyst at Emersion Insights.
Do you have a unique perspective on your research related to drug discovery and development? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net